Cardiovascular Topics
2023 Update Guidelines on Arterial Hypertension af European Society of Hypertension (ESH)
ESH har opdateret deres retningslinjer for behandling af arteriel hypertension med større vægt på brugen af klinisk valideret udstyr.

Vigtige budskaber
The ESH has recently updated their guidelines for the management of arterial hypertension, with greater emphasis on the use of clinically validated devices1
Home BP monitoring with validated devices leads to better blood pressure control, whereas the use of non-validated devices with inaccurate measurements can lead to misdiagnosis7
Hvad har ændret sig for blodtryksmålinger i de opdaterede ESH-retningslinjer fra 2023?1
Voksne
Opportunistic screening for all adults
Regular measurements for those >40 years or at high risk
Børn
Screening of all children <3 years old
Screening of children >3 years old with risk factors for high blood pressure (BP) including congenital heart disease, chronic kidney disease, solid organ transplantation, treatment with BP increasing drugs, or history of preterm birth
Enheder
Only properly validated devices should be used to ensure measurements are accurate
Automatic electronic, upper-arm cuff devices are recommended for both office and out-of-office use
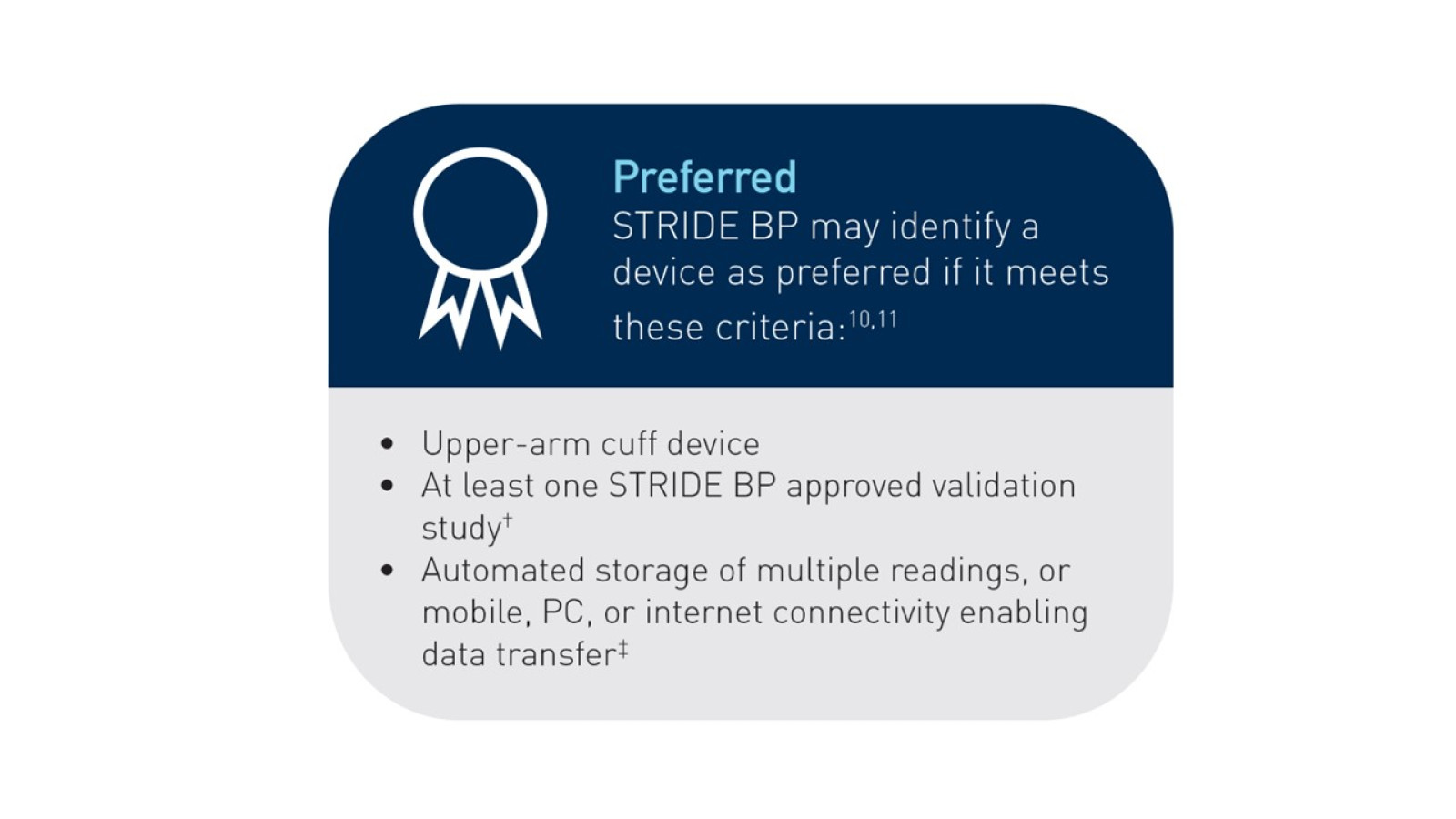
Devices with automated storage and connectivity are preferred
Support for telehealth technologies and nocturnal BP measurements are encouraged
Blodtryksmåling i hjemmet anbefales til:
Providing better reproducibility and prognostic value
Support the identification of white-coat or masked hypertension
Improving BP control through long-term follow-up of treated hypertension
Novel home devices could provide an alternative to ambulatory blood pressure measurement (ABPM) to measure blood pressure values during sleep to detect elevated or non-dipping blood pressure
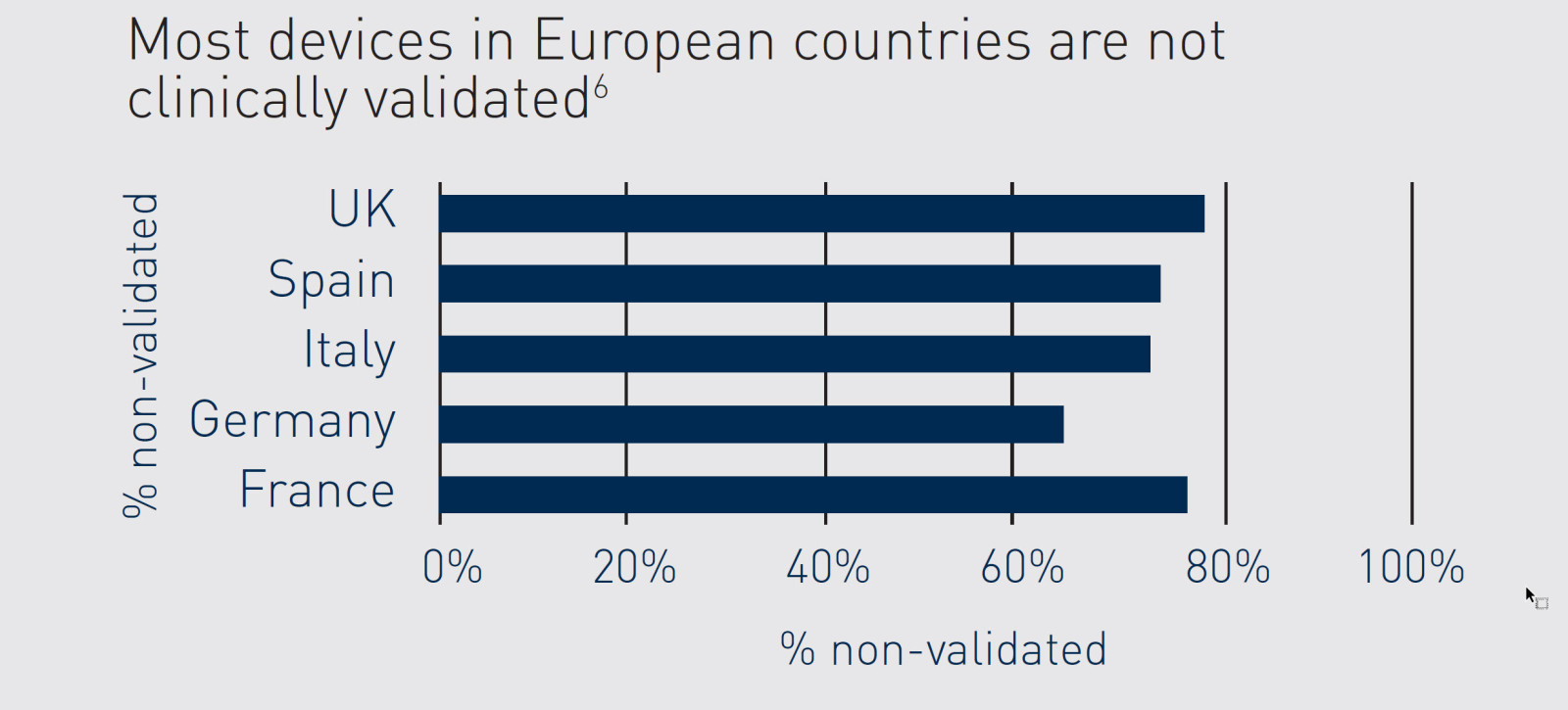
Hvorfor er klinisk validering af udstyr afgørende?
Selvom klinisk validering af nøjagtighed anbefales i retningslinjerne, er det ikke obligatorisk, før en blodtryksmåler (BPM) kommer på markedet3,4
På verdensplan er mindre end en fjerdedel af overarmsenhederne valideret eller svarer til en valideret enhed5
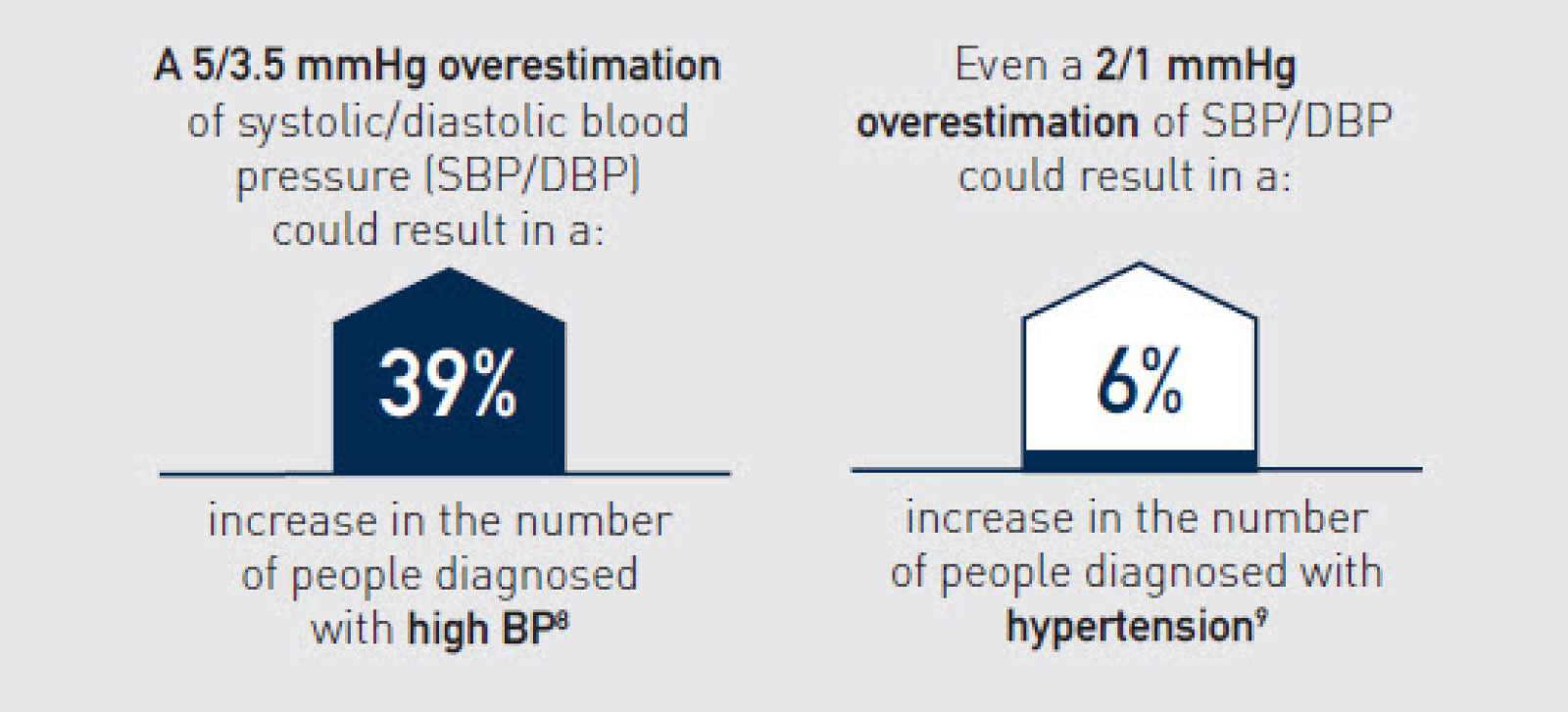
Ikke-valideret udstyr kan give unøjagtige målinger, hvilket fører til fejldiagnosticering og fejlbehandling⁷
ESH anbefaler, at sundhedspersonale, patienter og offentligheden tjekker STRIDE BP for at finde en liste over korrekt udstyr1
Lister over udstyr, der er valideret til brug i hjemmet, på kontoret/hospitalet eller i ambulatoriet, samt udstyr, der er valideret til brug hos børn eller gravide, findes på www.stridebp.org
STRIDE BP godkender i øjeblikket 390 af de >4000 enheder på markedet2

Hvordan valideres blodtryksmålere?
New devices are validated by comparing their measurements to those of reference devices using protocols set out by professional societies
A universal protocol has been developed by the US Association for the Advancement of Medical Instrumentation (AAMI), ESH, and the International Organization for Standardization (ISO)12
STRIDE BP will list a device if the results of such a validation study are published as a full paper10,11
Alle OMRON-enheder er klinisk validerede og listet af STRIDE BP13,14
OMRON ensures that every model of their BPMs meets the highest standards of precision and reliability13
OMRON has 91 validated upper-arm devices listed on STRIDE BP2

Referencer
1. Mancia G, et al. J Hypertens. 2023 juni 21.
3. Stergiou GS, et al. J Clin Hypertens (Greenwich). 2018 Jul;20(7):1096-9.
4. Picone DS, et al. Hypertension. 2020 Jun;75(6):1593-9.
5. Picone DS, et al. JAMA. 2022 Feb 15;327(7):680-1.
6. Picone DS, et al. JAMA. 2023 May 2;329(17):1514-6.
7. Stergiou GS, et al. JHypertens. 2021 Jul 1;39(7):1293-302.
8. Sakhuja S, et al. J Clin Hypertens (Greenwich). 2022 Mar;24(3):263-70.
9. Fan WG, et al. J Clin Hypertens (Greenwich). 2020 Feb;22(2):150-6.
10. https://stridebp.org/about-us/principles-for-device-listing. Tilgået 11/10/2023.
11. Stergiou GS, et al. J Clin Hypertens (Greenwich). 2019 Nov;21(11):1616-22.
12. Stergiou GS, et al. J Hypertens. 2018 Mar;36(3):472-8.
13. https://healthcare.omron.com/healthcare-solutions/cardiovascular-health/clinical-validations. Besøgt 23.10.18.
14. https://healthcare.omron.com/innovations/clinical-research-supporting-literature/cardiology/27/clinical-validation-of-medical-devices. Besøgt 18/10/2023.
† Udgivet inden for de seneste 10 år og ved hjælp af en nyere valideringsprotokol (AAMI/ESH/ISO 2018; ANSI/AAMI/ISO 2013 eller 2009; ESH-IP 2010).
‡ Dette kriterium gælder kun for apparater til hjemmebrug.
OHEAPP-345